Eli Lilly Halts Key Obesity Drug Combo Trial - $LLY Stock Impact
Pharma giant hits pause on high-stakes weight-loss treatment study.
Trial Termination Triggers Market Jitters
Eli Lilly abruptly stops development of its experimental obesity drug combination—sending ripples through healthcare portfolios. The sudden halt raises questions about the treatment's viability and competitive positioning against rival weight-loss therapies.
Pipeline Setback or Strategic Reset?
While the company maintains other obesity treatments in development, this specific combo drug represented a potential breakthrough approach. Analysts scramble to assess the broader implications for Lilly's metabolic disease franchise.
Wall Street's predictable panic reaction demonstrates how pharmaceutical investing remains a high-stakes gamble—where clinical trial results move markets faster than actual medical breakthroughs help patients.
TLDR
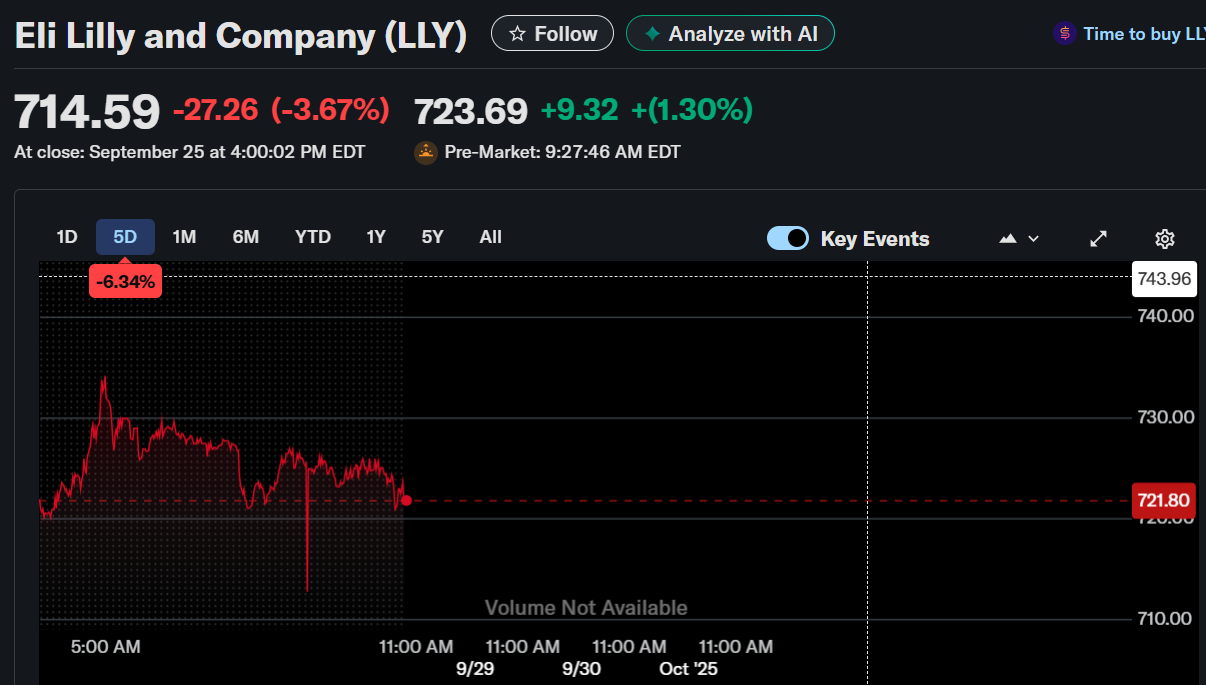
- Eli Lilly stock closed at $714.59, down 3.67% on September 25, 2025.
- The company halted a Phase 2 trial of bimagrumab with Zepbound.
- Decision cited “strategic business reasons,” not safety concerns.
- A separate trial in non-diabetic obese patients remains active.
- LLY shares are down 22% over the past year despite strong 5-year gains.
Eli Lilly and Company (NYSE: LLY) stock ended September 25 at $714.59, down 3.67%, before ticking up 1.14% in pre-market trading.
Eli Lilly and Company (LLY)
Shares slipped after the pharmaceutical giant halted a mid-stage clinical trial of bimagrumab, an experimental muscle-preserving drug it was testing with its blockbuster obesity treatment Zepbound.
The discontinued trial involved obese or overweight adults with type 2 diabetes. The company stated the decision was made for “strategic business reasons,” raising investor concerns about the future of muscle-preservation therapies in obesity treatment.
Development Background
Bimagrumab is designed to target proteins that regulate muscle growth, potentially preserving lean mass during rapid weight loss. Its relevance has grown as GLP-1 weight-loss drugs like Lilly’s Zepbound and Novo Nordisk’s Wegovy face scrutiny for reducing muscle along with fat.
Originally discovered by Novartis, bimagrumab was tested for rare muscle-wasting conditions before being licensed to Versanis Bio. Lilly acquired Versanis in 2023 for up to $1.9 billion, betting the drug could enhance Zepbound’s long-term value.
FDA and Industry Standards
The halted study, which was to enroll 180 patients and conclude in late 2026, ends at a time when the FDA is setting stricter standards. Regulators now suggest that muscle-preserving drugs must also demonstrate additive weight loss benefits when combined with GLP-1 treatments in order to secure approval.
A separate Phase 2 trial involving obese but non-diabetic patients continues recruiting, with initial results expected by April 2026. That study is measuring both muscle preservation and weight loss outcomes.
Competitive Landscape
Eli Lilly is not alone in pursuing muscle-sparing obesity treatments. Companies such as Regeneron, Biohaven, Scholar Rock, and Veru are also working on similar therapies. Veru’s stock fell earlier this week after disclosing that regulators now require incremental weight loss as a trial endpoint.
The evolving guidance highlights the challenges drugmakers face in extending the success of obesity drugs while addressing their limitations.
Performance Overview
Despite the pullback, Eli Lilly has outperformed over the long term. Year-to-date, LLY shares are down 6.88%, and they have declined 22.12% in the past year. However, over three years, the stock gained 135.26%, and over five years, it soared 402.97%, handily outpacing the S&P 500.

